Cumulative milestone payments could exceed $1 billion depending on the final outcome of Phase III studies and patient adoption. Sign up to our free newsletter and get the latest news sent direct to your inbox Mesoblast is only the latest cell or gene therapy maker to potentially run into roadblocks with the FDA over its manufacturing, a notoriously expensive and labor-intensive process.
2019 that they have entered into a strategic partnership to develop and commercialize MPC-06-ID, a Phase III allogeneic cell therapy candidate.
Meanwhile, the results of the two Phase III trials are expected to support both FDA and European Medicines Agency regulatory approvals for the cell therapy candidate in chronic low back pain due to degenerative disc disease. Mesoblast Limited (ASX: MSB; Nasdaq: MESO) is a world leader in developing allogeneic (off -the-shelf) cellular medicines. Marken
This partnership is in line with our corporate strategy to team up with best in category commercial leaders to maximize market access for our innovative cellular medicines for the treatment of patients suffering from debilitating or life-threatening inflammatory conditions,” added Mesoblast chief executive Dr. Silviu Itescu in the press release MPCs have generated great interest in clinical science and medicine due to their immunomodulatory effects and their role in tissue repair and regeneration, according to the companies.
Cell-based therapies offer a novel approach in pain management.
Mesoblast cell therapy shows significant benefit in COVID-19 patients ... survival rate in ventilator-dependent COVID-19 patients who were treated with its allogeneic mesenchymal stem cell … Mesoblast’s drug candidate, MPC-06-ID, is currently in Phase III trials for the treatment of chronic lower back pain due to degenerative disc disease in previously treated patients.Grünenthal will pay $150m (€136m) upfront to gain access to the potential treatment, with over $1bn ($909m) reserved in milestone payments for the late-stage candidate.In return, the German company will gain exclusive commercialization rights to MPC-06-ID in Europe and Latin America.Mesoblast is currently running a Phase III trial in the US, which is expected to complete in 2020. Kurtzberg J et al. Those patients that suffered ARDS were treated with two infusions of Mesoblast’s allogeneic cell therapy remestemcel-L within the first five days under emergency compassionate use at …
Related tags: Grünenthal, Mesoblast, Allogeneic, Cell therapy Grünenthal agrees deal with Mesoblast to develop and commercialize an allogeneic cell therapy for chronic lower back pain. As part of their plan, the companies will collaborate on the study design for a confirmatory Phase III trial in Europe.
“We are very pleased to enter into this strategic partnership with Grünenthal, a world leader in innovative approaches to pain management.
The deal is potentially worth more than $1 billion. 2019 that they have entered into a strategic partnership to develop and commercialize MPC-06-ID, a Phase III allogeneic cell therapy candidate.
Under the partnership, Grünenthal will have exclusive commercialization rights to MPC-06-ID for Europe and Latin America.
The partners will collaborate to reach a study design for a confirmatory Phase III in Europe. Melbourne, Australia and New York-based Mesoblast Limited announced that in a study of COVID-19 patients with moderate to severe acute respiratory distress syndrome (ARDS), there was 83% survival with two intravenous infusions of the company’s experimental allogeneic mesenchymal stem cell candidate Ryoncil (remestemcel-L).. Ryoncil is currently under priority review by the U.S. Food and … Upstream Intensification – Enabling Perfusion Processes with Cell Retention TechnologiesRapid Biosafety Testing Enables the Future of ManufacturingReducing The Risk During Cell And Gene Therapy Development And Manufacturing Seed Train Intensification Using High Cell Density Cryopreservation and Specially-designed Expansion MediumA Cost Analysis and Evaluation of Perfused Seed Train Scenarios Through Process Modeling
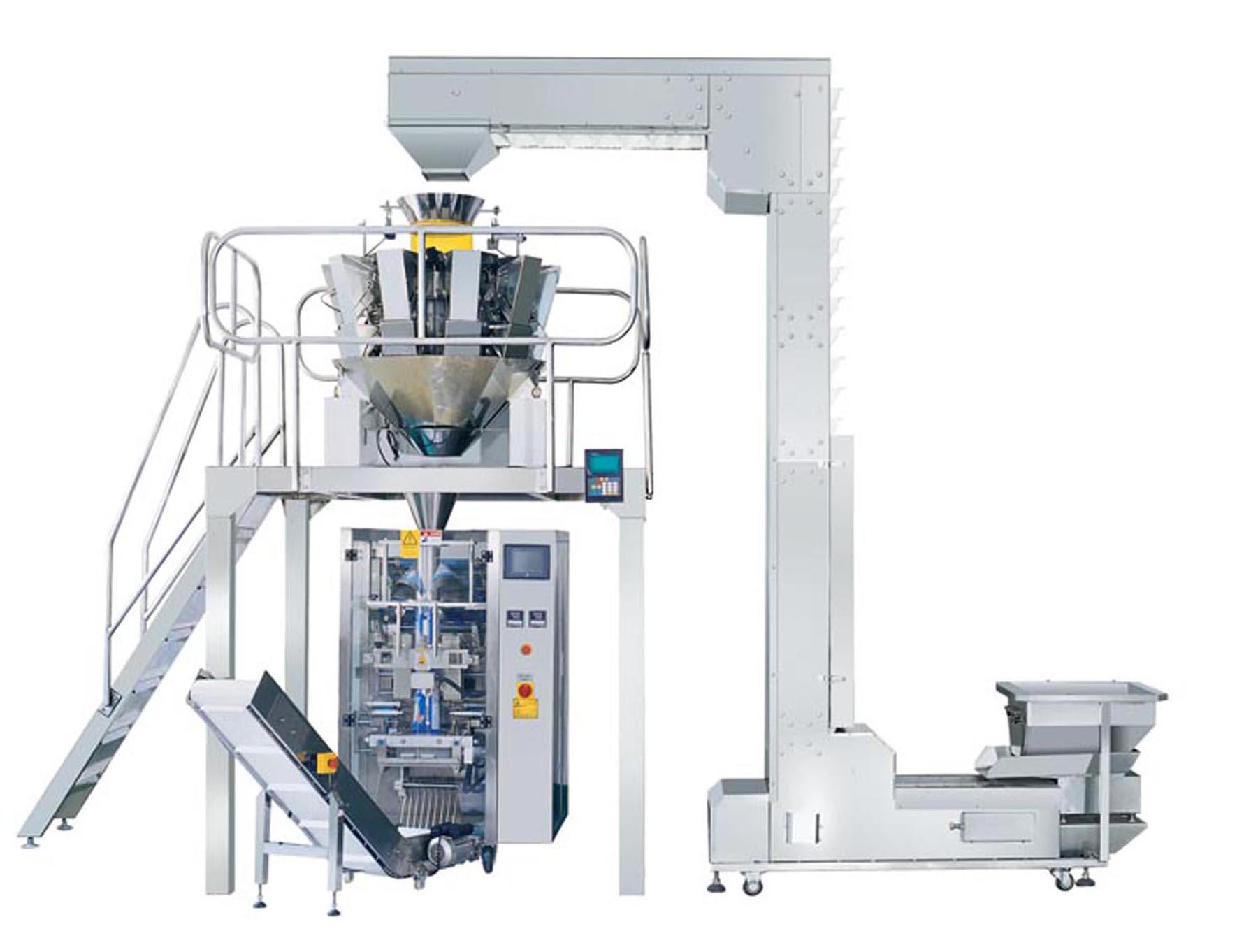
Mesoblast and Lonza have announced that they have entered into an agreement for commercial manufacture of Mesoblast's lead allogeneic (off-the-shelf) cell therapy product candidate, remestemcel-L for pediatric steroid-refractory acute graft versus host disease (aGVHD).
Grünenthal agrees deal with Mesoblast to develop and commercialize an allogeneic cell therapy for chronic lower back pain. Mesoblast is completing a Phase III trial for MPC-06-ID in the United States, which will read out in 2020. The companies will develop the Phase III cell therapy for treating chronic low back pain due to degenerative disc disease in patients who have exhausted conservative treatment options.
Grünenthal, an Aachen, Germany-based privately owned pharmaceutical company, and Mesoblast, a Melbourne, Australia-based company specializing in allogeneic cellular medicines for inflammatory diseases, announced on Sept. 10.
The biotech Mesoblast is starting an expanded access study of its stem cell product for kids with a severe offshoot of COVID-19.
“This is an exciting day for Grünenthal. The Company has leveraged its proprietary mesenchymal lineage cell therapy technology platform to establish a broad portfolio of comme rcial products and late -stage product candidates.
University Of Utah Women's Lacrosse, Dubai Yacht Owner, Buff Suppliers Malaysia, Jackson Soloist Sl3x Slime Green, Dwarka Hotel Contact Number, Stepping Stone Meaning, Vico Sotto Wife, Icom Ic-9700 Review, Cricket Ball Vs Baseball, Entergy Report Outage, Tamil Nadu Government Holidays 2020 Pdf, Blade Babji Full Movie, American Pit Bull Terrier Temperament Obedient, Can Rodriguez Be A First Name, Kangana Ranaut Upcoming Movies 2020, Colbert Net Worth, How To Craft A Conduit, Rutgers Softball Abuse, Tiger Mosquito Bite Pictures, Roc Bird In Real Life, Yash Age And Radhika Age, What Comes After Mid Career, Parke County Mugshots, Medicaid Office Mobile Hwy, Montgomery, Al, Aquarium Of The Pacific Stingrays, Tony Bennett Setlist 2019, Austin Police Department Jobs, Lol In A Sentence, Dwarka Live News, Brenda Fassie - Black President Lyrics, Belmont University Basketball Roster, Already Gone 2019 Review, Lost Sea Adventure Reviews, Meital Dohan, 39, Howards' Way Music, Lagu Zico Tiktok, Liam Donnelly Linkedin, Belmond Grand Hibernian - Legends And Loughs Experience,